
04 Nov A new era: Adding immunotherapy to surgical management of head and neck cancer
Written By: Ravindra Uppaluri, MD., PhD
Treatments have not changed for many years
For over two decades, the management of many locally advanced head and neck squamous cell carcinomas (HNSCCs) has involved up front surgery followed by radiation plus or minus chemotherapy. There have
been numerous advances in each of these modalities—surgeons are able to reconstruct complex surgical resections with advanced techniques, radiation is being delivered with more precision and different chemotherapy combinations have been developed. However, the clinical outcomes and toxicities of the multimodality therapy are not optimal. This is in particular true for human papilloma virus (HPV) unrelated HNSCCs of the oral cavity, larynx and hypopharynx. The 5-year survival for Stage III/IV HPV-unrelated HNSCCs is only approximately 50%. Patients also suffer from many different side effects including speech issues, dry mouth, fibrosis, and difficulty swallowing.
The last randomized Phase 3 trials that showed that any improvement in HNSCC outcomes were reported 21 years ago. These studies showed that high-risk patients, those whose resected tumor and lymph nodes showed positive surgical margins and/or extranodal extension of cancer cells in the tumor bearing lymph nodes, had improved outcomes when high dose cisplatin therapy was added to post-operative radiation therapy. Several clinical trials have tried to enhance these outcomes by adding other therapies but have failed to show improvements. Thus, the field and patients have been waiting for many years for news on changing the current standard of care.
Adding Immunotherapy
Over the last 15 years, many different cancer types have benefited by the introduction of therapies called immune checkpoint blockade. These therapies work by taking the “brakes” off immune cells, leading to immune cell activation and killing of tumor cells. These therapies (drugs called nivolumab and pembrolizumab) have been approved in HNSCC patients who have recurrent or metastatic disease.
We and others have explored the idea of whether addition of immune checkpoint blockade to standard of care surgically based HNSCC treatment would improve outcomes. My colleague Dr. Douglas Adkins at Washington University School of Medicine in St. Louis and I started a phase 2 study in 2015 to ask whether adding pembrolizumab before and after surgery would improve outcomes. Patients who were planned to undergo surgery received 1 or 2 doses of pembrolizumab before surgery. They then underwent standard surgery followed by pathology directed radiation -/+ chemotherapy
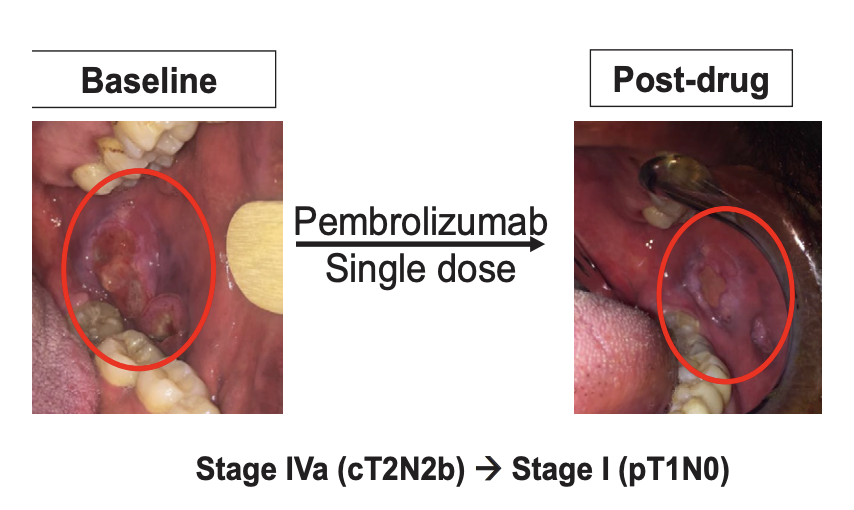
Interestingly, we found that patients had favorable clinical outcomes and we also saw pathologic tumor changes when patients received immunotherapy before surgery. In some patients, our pathologist saw dying tumor cells and increased immune cells in resected tumor and lymph nodes after just one dose of immunotherapy. Although rare, some patients had exceptional clinical response to the before surgery pembrolizumab (Figure 1). We also found that giving immunotherapy was well tolerated with minimal side effects and very importantly, did not alter planned surgery. After our study, several other studies also reported similar findings including using nivolumab and other immune checkpoint blockade therapies. All together these Phase 2 studies were encouraging that this could be a safe approach with a possibility of improved outcomes. This set the stage for the KEYNOTE 689 clinical trial.
Does perioperative immunotherapy improve HNSCC outcomes?
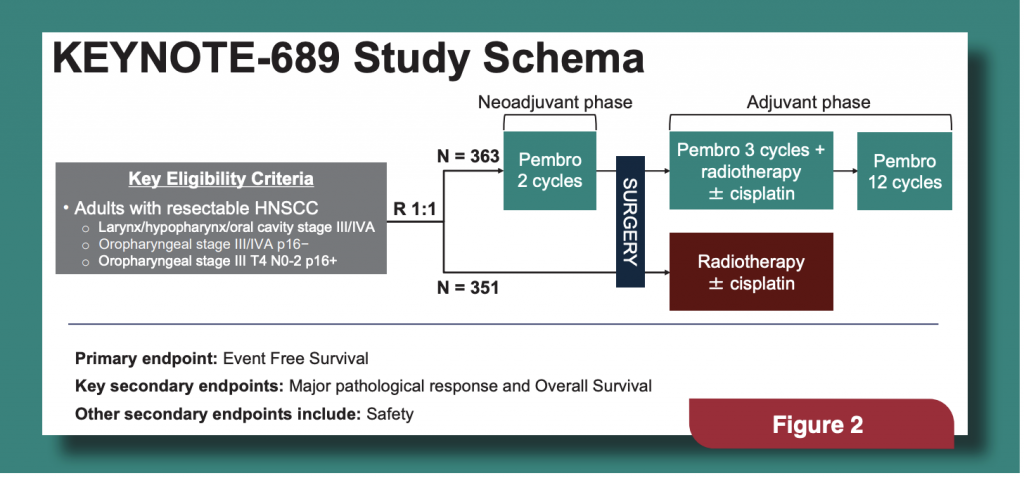
KEYNOTE-689, sponsored by Merck, was a phase 3 international clinical trial where Stage III/IV HNSCC patients were randomized to receive perioperative pembrolizumab (363 patients) or standard therapy alone (352 patients). All patients had newly diagnosed nonmetastatic locally advanced HNSCC that could be removed by surgery. The study schema is in (Figure 2).
The primary endpoint of the clinical trial was event free survival (EFS) defined as recurrence or death after the initial randomization. At a median follow-up of 38.3 months, EFS in patients in the pembrolizumab group compared to those treated with standard therapy alone, was 57.6% and 46.4%, respectively, representing a 27% reduction in the risk of disease progression, recurrence or death. Interestingly, highrisk features were seen in fewer patients who received pembrolizumab before surgery (32.5%) compared to the standard of care arm (44.4%). This led to the use of less radiation and chemotherapy in patients who received pembrolizumab. While there was a favorable trend in the overall survival of patients who received pembrolizumab, further data are needed to show this difference and this will be analyzed in the future.
Adverse events were similar between the two treatment groups, including the more concerning grade 3 or higher adverse events that occurred in 44.6% and 42.9% of patients in the pembrolizumab group and standard of care group, respectively. As expected, a higher percentage of patients in the pembrolizumab group had immune-mediated adverse events which were generally managed satisfactorily. Importantly, similar numbers of patients completed planned surgery in the pembrolizumab arm compared to the standard of care arm. These data support that giving pembrolizumab before surgery is safe without compromising patients’ ability to undergo planned surgery
Based on these data, the US Food and Drug Administration (FDA) approved this regimen for surgically resectable, locally advanced head and neck cancer in patients who have tumors with a combined positive score (CPS)≥1. The CPS score is determined based on testing of a tumor biopsy from the initial diagnosis of HNSCC and 95% of the patients in the KEYNOTE-689 trial were CPS≥1.
Conclusion
KEYNOTE-689 showed that adding perioperative pembrolizumab to standard of care HNSCC treatment reduced recurrence and death. This is an exciting time for HNSCC patients with the first change to the standard of care in over 20 years and this study also sets the stage for future advances in the field.
Disclosures
Dr. Uppaluri has served on advisory boards for Merck, Inc., Daiichi-Sankyo, Regeneron and Johnson and Johnson and has received research funding from Merck, Inc. and Daiichi-Sankyo.
